Indicaid Antigen 25 tests box
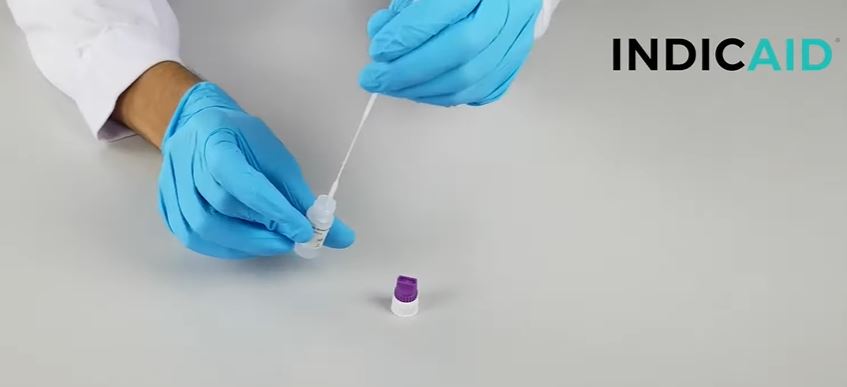
How to use the Indicate test?
- The kit is very easy to use. The nasopharingeal shwab can be brought less deep into the nose when you exhaust wet air. The liquid should be collected by the shwap.
- Today januray 2022 no saliva test kits are allowed by the FDA but we hope to have soon caliva test kits approved.
- The cassete detects the N capside of the virus that is present on the 2022 strains of Omicron and Delta.
- Results are within 15 minutes and not within 1 hour like for PCR. ( PCR however will detect 2 to 4 days earlier)
This Fact Sheet informs you of the benefits of the emergency use of the Atlas Link INDICAID Rapid Antigen Test.
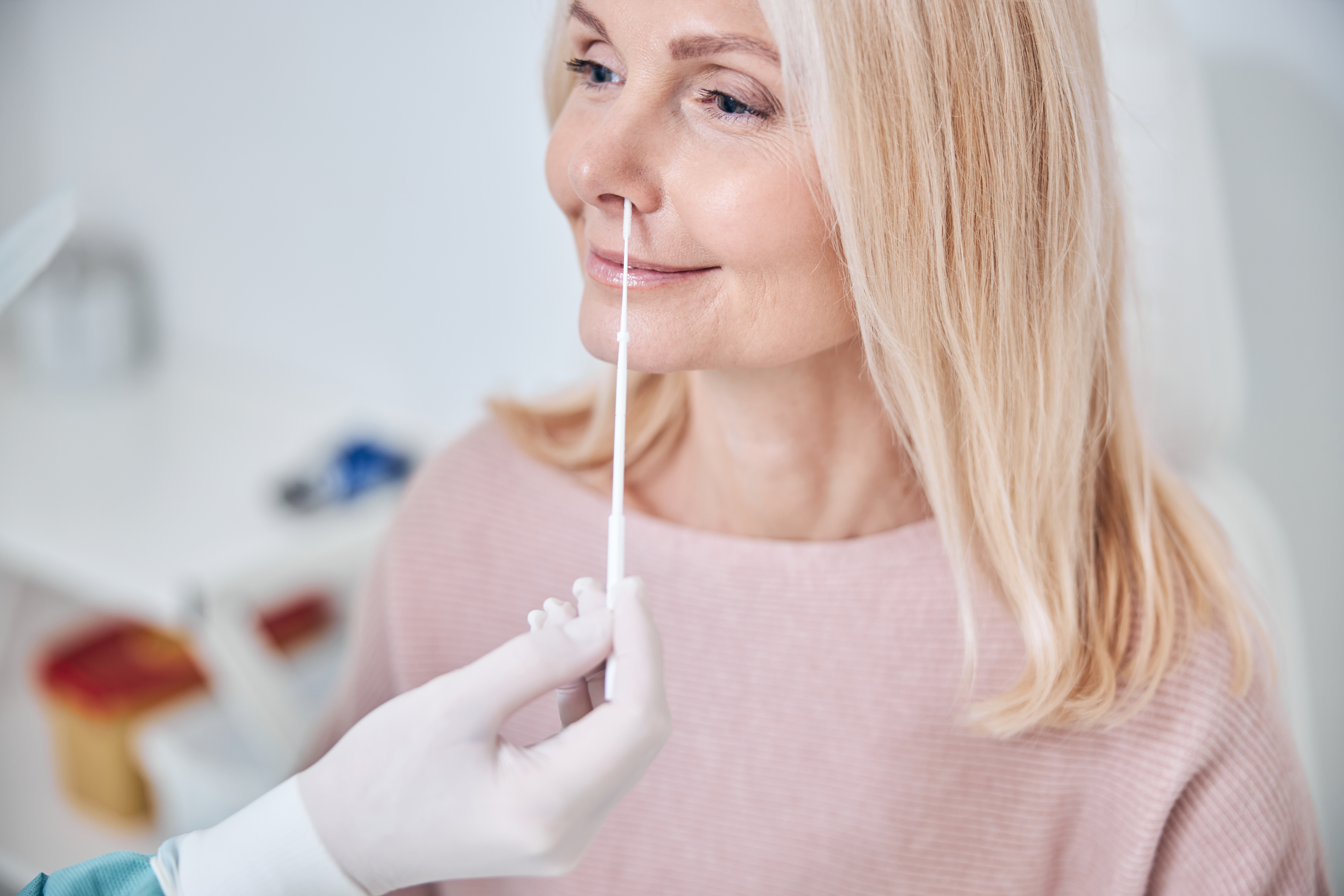
The Atlas Link Rapid Antigen Test is authorized for use using direct anterior nasal swab specimens from within the first five (5) days of signs of acute respiratory illness (e.g., cough, dyspnea) onset.
CURRENT INFORMATION ON COVID-19 FOR HEALTHCARE PROVIDERS IS AVAILABLE AT CDC’S WEBPAGE, INFORMATION FOR
HEALTHCARE PROFESSIONALS
- The INDICAID Rapid Antigen Test can be used to test direct anterior nasal swab specimens. Anterior nasal swab specimens may be collected by a healthcare provider (HCP) or selfcollected (by individuals 18 years of age or older, under the supervision of an HCP).
- The INDICAID Rapid Antigen Test should be ordered for the detection in individuals who are suspected of C-19 by their healthcare provider within the first five (5) days of symptom onset.
- The Atlas Link INDICAID Rapid Antigen Test is authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform moderate complexity, high complexity, or waived tests.
- The INDICAID C Rapid Antigen Test is authorized for use at the Point of Care (POC), i.e., in user care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. Please refer to the INDICAID Rapid Antigen Test Instructions for Use for additional information.
- Specimens should be collected with appropriate infection control precautions.
- Current guidance for collecting and handling specimens should be followed for individuals suspected of being infected.
- The CDC Interim Laboratory Biosafety Guidelines for Handling and Processing
Specimens
Rapid Antigen Test
This test is to be performed only using direct anterior nasal swab specimens from individuals by their healthcare
provider.
FACT SHEET FOR HEALTHCARE PROVIDERS
A positive test result indicates that nucleocapsid antigens present in all today known variants including Omicron were detected, and the patient is infected with the virus and presumed to be contagious.
Laboratory test results should always be considered in the context of clinical observations and epidemiological data (such as local Omicron outbreak/epicenter locations)
The INDICAID Omicron Rapid Antigen Test has been designed to minimize the likelihood of false positive test results.
All laboratories using this test must follow the standard testing and reporting guidelines according to their States appropriate public health authorities.
What does it mean if the specimen tests negative?
A negative test result for this test means that nucleocapsid antigens from SARS variant were not present in the specimen above the limit of detection.
However, a negative result does not rule out a latent infection and should not be used as the sole basis for decisions
Antigen tests are known to be less sensitive than molecular PCR tests that detect viral nucleic acids but they can be done dayly to monitor large groups of persons.
The amount of antigen in a sample may decrease as the duration of illness increases. Specimens collected after day 5 of illness may be more likely to be negative compared to a RT-PCR assay. Therefore, negative results from patients with symptom onset beyond 5 days should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management.
It is possible to test a person too early or too late to make an accurate diagnosis via the
INDICAID Rapid Antigen Test?
Yes it is absolutely possible when the testing is negative, the possibility of a false negative result should be considered in the context of a patient’s recent exposures and the presence of clinical signs and symptoms.
Risks to a patient from a false negative result include to early or to late testing.
For additional recommendations regarding infection control, refer to CDC’s Discontinuation of
Isolation
Where do I report Adverse events, including problems with test performance or results?
Your complaints should be reported to to MedWatch by submitting the online FDA Form 3500
(https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home) or by calling 1-800-FDA-1088
The performance of the Atlas Link Indicate test was established based on the evaluation of a limited number of clinical specimens collected between February 2021 and March 2021. The clinical performance has not been established in all circulating delta and omicron variants but is anticipated to be reflective of the less mutating N capside present in Omicron (B.1.1.529): Variant of Concernfor2022.
