Description
INTENDED USE This product is used for in vitro qualitative detection of novel coronavirus (SARS-CoV-2) antigen in human oropharyngeal swabs, nasal swabs and nasopharyngeal swabs. This product is used under medical institutions only. The SARS-CoV-2 is a new type of coronavirus and named by the World Health Organization.
The SARS-CoV-2 has spread all over the world. It causes viral pneumonia with fever, fatigue, dry cough and sore throat as the main manifestations. The severe cases of viral pneumonia caused by it manifested as dyspnea, decreased blood oxygen saturation, and rapid development of acute respiratory distress syndrome, septic shock, etc. In serious cases, metabolic acidosis and coagulation dysfunction are difficult to be treated, which directly affect life and health.
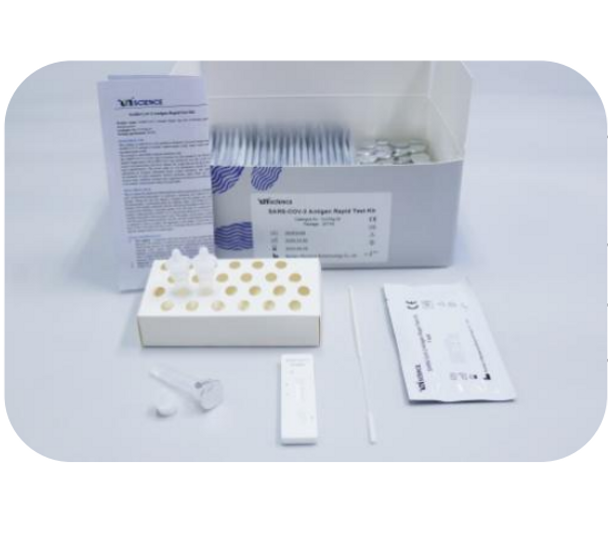
UnScience Antigen Test (CoV2Ag-25)

TEST PRINCIPLE
This kit adopts the sandwich method and the technical principle of colloidal gold immunochromatography to qualitative determine the SARS-CoV-2 antigen. During the test, the sample is dropped into the sample well, and chromatography is performed under the capillary effect. The SARS-CoV-2 antigen in the sample combined with the colloidal gold-labeled SARS-CoV2 monoclonal antibody I, and then spread to the test area.
It is captured by another coated antibody (SARS-CoV-2 monoclonal antibody II), to form a complex and gather in the test area (T line). The quality control area is coated with the goat anti-mouse antibody, and the colloidal gold-labeled antibody is captured to form a complex and aggregate in the quality control area (C line). If the C line does not show color, it indicates that the result is invalid, and this sample needs to be tested again.
MAIN COMPONENTS
1. Test reagent: 1 test/pouch, each test consists of a test cassette and a desiccant. The cassette is composed of a test strip and a test strip shell. The test strip consists of a sample pad and a colloidal gold bonding pad (sprayed with colloid Gold-labeled SARS-CoV-2 monoclonal antibody I), nitrocellulose membrane (NC membrane) (the detection area is coated with SARS-CoV-2 monoclonal antibody II (T line) and goat anti- Mouse IgG (C line)), liner and absorbent pad. 2. Desiccant: 1 piece/pouch, silica gel. 3. Swab: 25 pieces/pack. 4. Sample treatment solution: 25 vials/pack. 5. Tube cap: 25 pieces/pack.
STORAGE AND STABILITY
The test reagent is stored at 2℃-30℃, and the validity period is tentatively set for 18 months. See the label for the production date and expiration date.
SAMPLE REQUIREMENTS
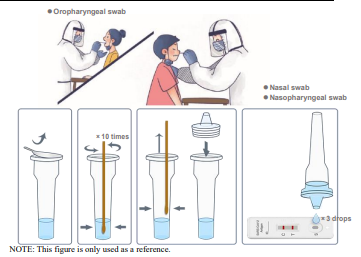
1. Oropharyngeal swab: The head of the person is slightly tilted, with mouth wide open, exposing the pharyngeal tonsils on both sides. Use the swab to gently wipe the tonsils on both sides for at least 3 times, and then wipe the posterior pharyngeal wall up and down at least 3 times. 2. Nasal swab: Prior to collecting the nasal swab, the patient should be instructed to blow their nose.
Carefully insert the swab into the nostril with the most secretion under visual inspection. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril), and rotate the swab against the nasal wall several times and then remove it from the nostril. 3. Nasopharyngeal swab: Carefully insert the swab into the nostril with the most secretion under visual inspection.
Keep the swab near the septum floor of the nose while gently pushing the swab into the posterior nasopharynx. Rotate the swab several times then remove it from the nasopharynx (in case of reflex cough, stop for 1 minute).
Additional Information
SIZE: |
25 Tests/Kit |
SENSITIVITY: |
90.323% |
SPECIFICITY: |
99.213% |
BROCHURE: |